- Your cart is empty
- Continue Shopping

Product
Product Description
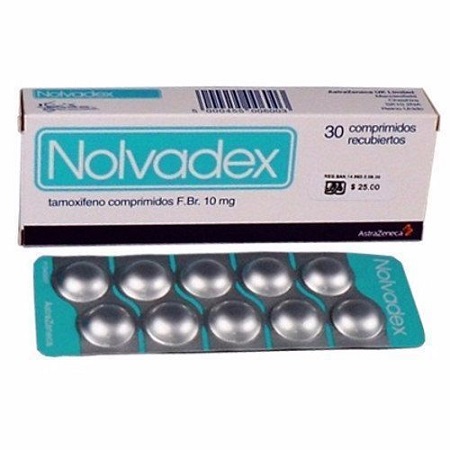
Buy Nolvadex 10mg Online | Order Nolvadex Online | Nolvadex
Nolvadex 10mg is used to treat patients with breast cancer, the recommended daily dose is 20-40 mg. Dosages greater than 20 mg per day should be given in divided doses (morning and evening).
In three single agent adjuvant studies in women, one 10 mg NOLVADEX (tamoxifen citrate) tablet was administered two (ECOG and NATO) or three (Toronto) times a day for two years. In the NSABP B-14 adjuvant study in women with node-negative breast cancer, one 10 mg NOLVADEX (tamoxifen citrate) tablet was given twice a day for at least 5 years. Results of the B-14 study suggest that continuation of therapy beyond five years does not provide additional benefit (see CLINICAL PHARMACOLOGY). In the EBCTCG 1995 overview, the reduction in recurrence and mortality was greater in those studies that used tamoxifen for about 5 years than in those that used tamoxifen for a shorter period of therapy. There was no indication that doses greater than 20 mg per day were more effective. Current data from clinical trials support 5 years of adjuvant NOLVADEX (tamoxifen citrate) therapy for patients with breast cancer.Nolvadex 10mg
Ductal Carcinoma in Situ (DCIS)
The recommended dose is NOLVADEX (tamoxifen citrate) 20 mg daily for 5 years.
Reduction in Breast Cancer Incidence in High Risk Women
The recommended dose is NOLVADEX (tamoxifen citrate) 20 mg daily for 5 years. There are no data to support the use of NOLVADEX (tamoxifen citrate) other than for 5 years (See CLINICAL PHARMACOLOGY-Clinical Studies – Reduction in Breast Cancer Incidence in High Risk Women).Nolvadex 10mg
HOW Nolvadex 10mg is SUPPLIED
10 mg Tablets containing tamoxifen as the citrate in an amount equivalent to 10 mg of tamoxifen (round, biconvex, uncoated, white tablet identified with NOLVADEX (tamoxifen citrate) 600 debossed on one side and a cameo debossed on the other side) are supplied in bottles of 60 tablets. NDC 0310-0600-60.
20 mg Tablets containing tamoxifen as the citrate in an amount equivalent to 20 mg of tamoxifen (round, biconvex, uncoated, white tablet identified with NOLVADEX (tamoxifen citrate) 604 debossed on one side and a cameo debossed on the other side) are supplied in bottles of 30 tablets. NDC 0310-0604-30.
SIDE EFFECTS of Nolvadex
Adverse reactions to NOLVADEX (tamoxifen citrate) are relatively mild and rarely severe enough to require discontinuation of treatment in breast cancer patients.
Continued clinical studies have resulted in further information which better indicates the incidence of adverse reactions with NOLVADEX (tamoxifen citrate) as compared to placebo.
Metastatic Breast Cancer
Increased bone and tumor pain and, also, local disease flare have occurred, which are sometimes associated with a good tumor response. Patients with increased bone pain may require additional analgesics. Patients with soft tissue disease may have sudden increases in the size of preexisting lesions, sometimes associated with marked erythema within and surrounding the lesions and/or the development of new lesions. When they occur, the bone pain or disease flare are seen shortly after starting NOLVADEX (tamoxifen citrate) and generally subside rapidly.
In patients treated with NOLVADEX (tamoxifen citrate) for metastatic breast cancer, the most frequent adverse reaction to NOLVADEX (tamoxifen citrate) is hot flashes.
Other adverse reactions which are seen infrequently are hypercalcemia, peripheral edema, distaste for food, pruritus vulvae, depression, dizziness, light-headedness, headache, hair thinning and/or partial hair loss, and vaginal dryness











Reviews
There are no reviews yet.